Abstract
Mantle cell lymphoma (MCL) remains incurable due to the development of drug resistance, despite the plethora of therapies available. Each successive treatment failure is associated with a more rapidly proliferating disease and fewer treatment options. Understanding the drug-resistance mechanism and developing effective, well-tolerated, new therapies for MCL are urgently needed.
Unrestrained proliferation of MCL cells is mainly driven by aberrant cyclin D1 expression and dysregulated CDK4 activity that promotes cell cycle progression from G1 to S. In our phase 1 clinical trial inhibiting CDK4/6 with palbociclib and BTK with ibrutinib (PALIBR) in recurrent MCL, the complete response rate (CR) was 42% compared to 21% in response to ibrutinib alone, despite a comparable 67% overall response rate. Moreover, 5 patients remain on therapy (2 CR and 3 PR) for ~ 8 years, suggesting that CDK4/6 inhibition deepens and prolongs the clinical response to BTK inhibition in MCL.
However, resistance emerges in some patients. To elucidate the underpinnings, we undertook integrative longitudinal genomic analysis of sequential tissue and blood specimens (n=53) of 27 MCL patients before, during therapy and on progression, and 4 treatment-naïve MCL patients and 4 normal subjects as controls. Whole exome and whole transcriptome sequencing of freshly isolated MCL cells and B cells revealed that resistance to PALIBR is rooted in homozygous deletion of RB1 (direct target of CDK4/6) or compound copy number variation (cCNV) - concurrent hemizygous loss of RB1 and CDKN2A (p16, CDK4/6 inhibitor) and gain of CDK4. In other resistant patients, the RB protein is silenced by translational termination mutation.
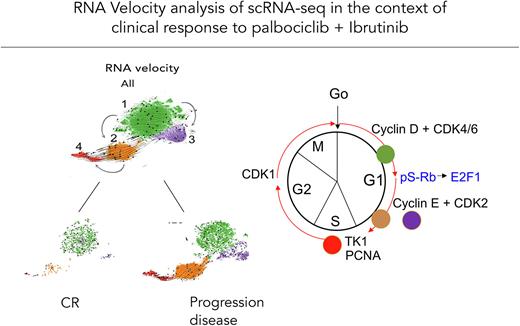
Single-cell RNA-sequencing (scRNA-seq, 210,000 cells) of corresponding PBMCs using a unique in-house MCL RNA reference library reveals that MCL cells comprise 4 transcriptomically distinct clusters. Cluster 1 (C1) is similar to quiescent normal B cells; C2 resembles hyper-activated B cells enriched for signatures of BCR and cytokine signaling; C3 represents non-proliferating, long-lived MCL cells; and C4 is highly proliferative, expanding with disease progression.
RNA velocity analysis further uncovered that C2 fuels the transition to C4 with disease progression, whereas C3 is a terminal node (Fig 1). In C2 and C3 MCL cells, CDK2 is highly elevated, indicating evasion of CDK4/6 inhibition, and PIK3IP1 (negative PI3K regulator) is silenced, suggesting that compensatory PI3K activation in BTKi resistance (Chiron et al, Cancer Discovery 2014) is secondary to loss of cell cycle control. BCL2 is markedly elevated in C2, greater in C3 MCL cells along with increases in anti-apoptotic BCL2L1(BCL-XL) and BCL-2A1 and decreases in pro-apoptotic PMAIP1 (NOXA) expression. This suggests combined CDK2 and BCL2 inhibition as a rational approach to override CDK4/6i and BTKi resistance.
Indeed, combined inhibition of CDK2 with PF-07104091 and BCL2 with venetoclax (PF-VEN) cooperatively impaired growth and killed ibrutinib-resistant MAVER-1 cells, more effectively in the isogenic derivative MAVER-1R cells which we generated to recapitulate cCNV in PALIBR resistance - depletion of RB protein through translational termination mutation (W99, AAF 75%) in RB1, loss of CDKN2A and gain of CDK4. Mechanistically, MAVER-1R cells are accelerated in G1 to S progression, based on elevation of CDK2 and TK1 expression and cell cycle profiling. They are intrinsically apoptotic and vulnerable to BCL2 inhibition due to a marked reduction in cells expressing anti-apoptotic BCL2L1 and IRF4, and universal expression of pro-apoptotic PMAIP1 (NOXA). Importantly, combined CDK2 and BCL2 inhibition synergistically kills MCL cells of a patient resistant to PALIBR ex vivo.
In summary, by longitudinal genomic analysis, we have provided the first evidence that 1) resistance to CDK4/6i and BTKi stems from MCL intrinsic cCNV; 2) MCL cells comprise 4 major transcriptomically distinct clusters; cluster 2 expressing copious CDK2 and BCL2 is pivotal in fueling proliferation of MCL cells in resistance; 3) cluster 3 cells also accumulate with resistance due to longevity; 4) combined inhibition of CDK2 and BCL2 overrides resistance to CDK4i and BTKi, in MCL cell lines and primary MCL cells from PALIBR resistant patient. Combined inhibition of CDK2 and BCL2, therefore, represents a mechanism-based strategy to overcome CDK4/6i and BTKi resistance in MCL therapy.
Disclosures
Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Elemento:AstraZeneca: Research Funding; Janssen: Research Funding; Volastra Therapeutics: Consultancy, Current equity holder in publicly-traded company, Research Funding; Johnson and Johnson: Research Funding; Eli Lilly: Research Funding; Freenome: Consultancy, Other: Current equity holder in a privately-held company; Owkin: Consultancy, Current equity holder in publicly-traded company; One Three Biotech: Consultancy, Current equity holder in publicly-traded company; Champions Oncology: Consultancy.
OffLabel Disclosure:
Venetoclax is a BCL2 inhibitor FDA-approved for chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and acute myeloid leukemia (AML). It was used off-label in combination with ibrutinib in a phase II clinical trial in patients with mantle cell lymphoma. Palbociclib is a CDK4/6 inhibitor FDA-approved for breast cancer. It was used off-label in combination with ibrutinib in a phase I clinical trial in patients with relapsed/refractory mantle cell lymphoma. Also, PF-07104091 is a CDK2 inhibitor that is used for preclinical study only.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal